9. Thermodynamic Equilibrium:
Chemical equilibrium, kinetics, and thermodynamics
1st law of thermodynamics
The first law of thermodynamics is a thermodynamic adaptation of the law of conservation of energy that distinguishes between three types of energy transfer: heat, thermodynamic work, and energy associated with matter transfer.
It also relates each type of energy transfer to a property of a body's internal energy.
It is common to formulate the first law for a thermodynamic process without a transfer of substance as follows:
ΔU=Q-W
Q > 0: system take up
heat
Q < 0: system releases heat
W > 0: system does work
W < 0: work is done to the system
ΔU > 0: system takes up energy
ΔU < 0: system loses energy
A thermodynamic system's enthalpy, which is one of its properties, is calculated by adding the system's internal energy to the product of its pressure and volume.
The pressure-volume concept describes the effort needed to create space for the system by displacing its surrounds in order to determine its physical dimensions.
Enthalpy, then, serves as a stand-in for energy in chemical systems; bond, lattice, solvation, and other properties that we refer to as "energies" in chemistry are actually differences in enthalpy.
Enthalpy as a state function depends simply on the ultimate arrangement of internal energy, pressure, and volume, not on the procedure followed to get there.
ΔH = ΔU + pΔV
(by changing Q in U = Q - W to H)
Enthalpy H is a state function with the following definition: H=U+pV.
2nd Law of Thermodynamics
"The entropy will rise in a spontaneous (naturally occurring) process"
We must always take the environment and system entropy changes into account.
ΔStot = ΔSsys + ΔSenv
Gibbs Free energy
ΔSenv = ΔH/T
ΔStot = ΔSsys -ΔH/T
TΔStot = TΔSsys-ΔH
Substitute ΔG for -TΔS : ΔG = ΔH-TΔSsys
ΔG = ΔH-TΔS changes in gibbs free energy
G = H-TS gibbs free energy
Meaning of ΔG :
G is like H and S- a state function ΔG = G2-G1
Finally, the Gibbs free energy function allows us to determine whether a process will occur spontaneously under specific conditions (temperature and pressure constant)
ΔG > 0 reaction will not happen spontaneously.
ΔG = 0 System is in thermodynamic equilibrium.
ΔG < 0 reaction will happen spontaneously.
Chemical Equilibrium
The majority of chemical processes and reactions
are reversible.
Thermodynamic equilibrium is the end stage of all reversible processes.
Equilibrium is established in a chemical reaction when the two opposing
reversible reactions happen at the same reaction rate.
When a reaction happens, the opposite reaction will also happen:
N2(g)
+ 3H2(g)
=====> 2NH3(g)
2NH3(g)
====> N2(g)
+ 3H2(g)
A double arrow is used to denote this.
N2(g) + 3H2(g) <====> 2NH3(g)
When the two opposing reversible reactions happen at the same rate, equilibrium has been attained.
A2(g) + X2(g) = 2AX(g)
The change in substance concentration over time is referred to as the rate of a chemical reaction, or r.
r=dc(X)/dt
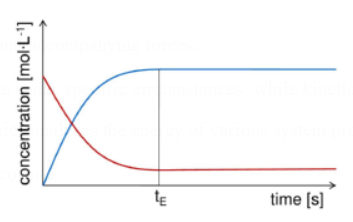
All substances have a constant concentration while a chemical reaction is in equilibrium.
Due to the equal rates of the forward and backward reactions, there is no overall change in the reactions.
There is a dynamic equilibrium in the system.
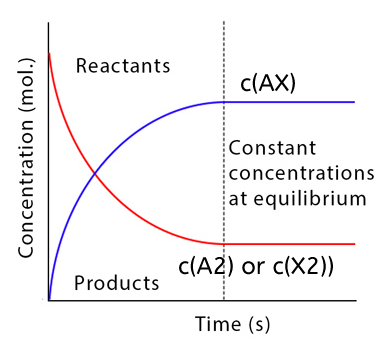
Figure 1. Reactant and product concentrations in a equilibrium reaction.
We may calculate the forward and reverse reaction's rates as follows if they are single steps:
r1 = kf *c(A2).c(X2)
r1 = kr * c2(AX)
In the state of equilibrium :
r1
= rr
kf * c(A2) * c(X2)
= kr*c2(AX)
c2(AX)/c(A2) * c(X2) ====> kf/kr
Equilibrium constant
The Law of Mass Action
aA+eE = xX+zZ
k=cx(X) * cz(Z)/ca(A)*cc(C)
The ratio between the product of the product concentrations and the product of the reactant concentrations is constant.
The Equilibrium Constant Kc
The law of mass action is stated for concentrations for reactions of gases and in solutions. The index c in 'KC' indicates this.
The composition of the system at equilibrium in terms of educts and products is shown by the value of "KC"
Net substance changes will continue until the ratio Q of products to byproducts equals KC, at which point the system will have attained equilibrium.
Q < Kc: net foward reaction will
occur
Q = Kc: equilibrium
Q > Kc: net reverse reaction will occur
The Equilibrium Constant Kp
The ideal gas law links concentration and (partial) pressure.
p = n/V . RT = cRT
As a result, we can formulate the law of mass action with partial pressures for reactions that only involve gases:
aA(g) + eE(g) = xX(g) + zZ(g)
Kp=px(X).pz(Z)/pa(A).pe(E)
Le Chatelier’s Principle
If a chemical
reaction is at equilibrium and experiences a change in pressure,
temperature, and concentration of product or reactant, the equilibrium
shifts in the direction that tends to undo the change.
1. effect of concentration :
If we increase the reactant, the reaction will
proceed to produce more product, hence it will move in the
forwarding direction.
If we increase the product, the reaction will proceed to produce more
reactants, hence it will move in the
backward direction.
2. effect of pressure :
As pressure is directly proportional to the
number of moles
As we increase pressure, the reaction
will move where there are fewer moles.
3. effect of addition of inert gas:
At constant pressure: equilibrium will shift
towards more number of gas moles as it will
increase the vessel`s volume
At constant volume: it will not affect the equilibrium.
4. effect of temperature:
On increasing,
temperature equilibrium will shift
towards an endothermic reaction.
Exothermic reactions are favored at low temperature
Endothermic reactions are favored at high temperature
5. effect of catalyst :
It doesn't affect the equilibrium
It only increases the rate of reaction and decreases the activation
energy of a reaction.
eg:
H2+I2⇒ 2HI+ heat
1. If we increase the product
concentration , the reaction will move in the backward direction.
2. If we increase the pressure, the reaction will have no effect as the
number of moles on both sides is equal.
3. If we increase the temperature, the exothermic reaction will move in
a backward direction producing more reactants.
4. If we add a catalyst, the equilibrium will not change.
A new equilibrium is established when a system in equilibrium is subjected to an imposed change in concentration, temperature, volume, or partial pressure.
Example:
2S02(g) + 02(g) <===> 2S03(g)
Since 3 moles of gas are reduced to 2 moles under higher pressure, the fon/vard reaction is more favorable.
If there is an exothermic reaction and a rise in temperature, how do you think the equilibrium will change?
Temperature-Dependence of Equilibrium Constants
Le Chatelier's idea is quantitatively expressed in the previously mentioned standing equation.
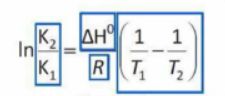
The expression on the right side of the equation is negative if T2 > T1 and the reaction is exothermic (ΔH <O).
K2 <K1 as a result, and with the higher temperature T2, the reactant side is where the equilibrium is now.
Thermodynamics vs. Kinetics
The study of energy changes using
thermodynamics.
Science of kinetics that examines the
motions/velocities of particles and accompanying forces.
Thermodynamics predicts whether a chemical reaction will take place
under specific circumstances, while kinetics assesses the rate of this
reaction.
While kinetics evaluates the speed at which
states shift, thermodynamics analyzes the energy of various system
process stages.
When chemical equilibrium is created, thermodynamics and kinetics
collide.
Chemical Equilibrium
Differences in free energy between states are used in thermodynamics to establish equilibrium concentrations (reactants vs. products)
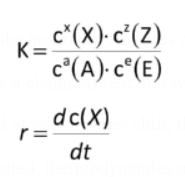
Boltzmann distribution : Probability as a function of energy of a specific state (substance)
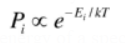
Pofential Energy Surface
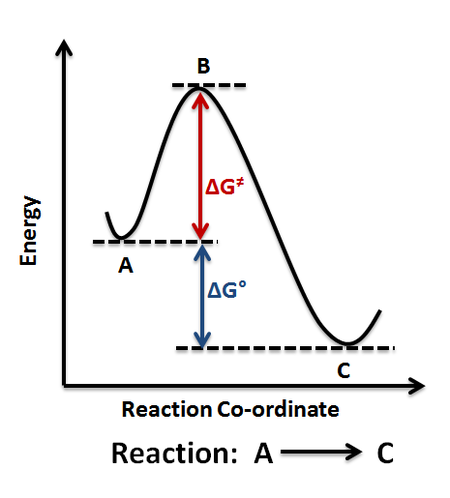
A potential energy surface, often known as a PES, is a mathematical or visual representation of the relationship between a molecule's energy and shape.
---- Summary ----
As of now you know all basics of all the laws of thermodynamics.
-
Gibbs free energy
-
Chemical equilibrium
-
Thermodynamics vs Kinetics
-
etc..
________________________________________________________________________________________________________________________________

________________________________________________________________________________________________________________________________
