8. Thermodynamics:
Thermochemistry
A substance change in a chemical reaction is
typically accompanied by an energy change.
The energy that is released or absorbed can take on various forms,
including heat but also light, electric energy, mechanical energy, and
others.
The study of energy change—expressed as heat—associated with chemical
changes is known as thermochemistry.
Measuring Energy
Newton's second law of motion in classical mechanics gives us the
following relationship between a force F and the acceleration a that a
body of mass m experiences:
F=ma
In Newtons, force is measured (N).
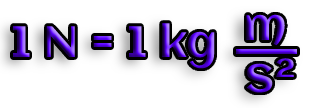
A mass of 1 kg moving at 1 m/s^2 requires a force of 1 Newton:
The dot product of a force F and the work W is referred to as
W=F-d
the displacement of an item by the given distance d.
In Joules, work is measured (J).
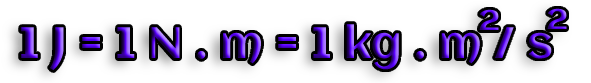
The labor required to apply a force of 1 N over a distance of 1 m is measured in units of joules:
A body of mass m has kinetic energy Ekin if it
is accelerated to a velocity of v using the work W.
The kinetic energy is connected to mass and velocity and equivalent to
the work W:
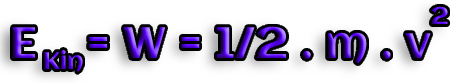
Consequently, the Joule is the unit of kinetic energy (J)
Conversation of energy
Energy cannot be created or destroyed in a
closed system, but it can be changed from one form to another.
When friction slows a moving object with kinetic energy
(Ekin), the kinetic
energy is converted to heat until the object comes to rest.
Independent of the specific form it takes, energy is measured in Joules.
Heating and Temperature
Every object has the potential to have thermal
energy. At T > 0K, the energy is connected to the random motion of
molecules.
If two things in thermal contact are at different temperatures, they can
exchange energy as heat. The system eventually finds equilibrium, and
its macroscopic properties remain unchanged.
In Kelvin [K], temperature is measured.
or in degrees Celsius [°C], where x K = (x - 273.15) °C
Heat capacity
Heat capacity C [J/K]
: The quantity of heat/energy (1J) required to raise an object's temperature by 1K;
[J/K].
Specific Heat Capacity [J/gK] :
The amount of energy(1J) required to raise 1g of a substance's temperature
by 1K is known as its specific heat (or capacity; unit: [ J/gK ]).
In the past, a heat-based energy unit called the calorie was defined
using the specific heat capacity of water.
1 cal = 4.184 J
| Substances | Phase | Specific heat capacity |
|
Air (Sea
level, dry, 0 °C (273.15 K)) |
gas | 1.0035 |
|
Air (typical room conditionsA) |
gas | 1.012 |
| Aluminium | solid | 0.897 |
| Ammonia | liquid | 4.7 |
| Animal tissue(incl. human) | mixed | 3.5 |
| Antimony | solid | 0.207 |
| Argon | gas | 0.5203 |
| Arsenic | solid | 0.328 |
| Beryllium | solid | 1.82 |
| Bismuth | solid | 0.123 |
| Cadmium | solid | 0.231 |
| Carbon dioxide CO2 | gas | 0.839 |
| Chromium | solid | 0.449 |
| Copper | solid | 0.385 |
| Diamond | solid | 0.5091 |
| Ethanol | liquid | 2.44 |
| Gasoline (octane) | liquid | 2.22 |
| Glass | solid | 0.84 |
| Gold | solid | 0.129 |
| Granite | solid | 0.79 |
| Graphite | solid | 0.71 |
| Helium | gas | 5.1932 |
| Hydrogen | gas | 14.3 |
| Hydrogen sulfide H2S | gas | 1.015B |
| Iron | solid | 0.412 |
| Lead | solid | 0.129 |
| Lithium | solid | 3.58 |
| Lithium at 181 °C | solid(?) | 4.233 |
| Lithium at 181 °C | liquid | 4.379 |
| Magnesium | solid | 1.02 |
| Mercury | liquid | 0.1395 |
| Methane at 2 °C | gas | 2.191 |
| Methanol | liquid | 2.14 |
| Molten salt (142–540 °C) | liquid | 1.56 |
| Nitrogen | gas | 1.04 |
| Neon | gas | 1.0301 |
| Oxygen | gas | 0.918 |
|
Paraffin wax C25H52 |
solid | 2.5 (ave) |
|
Polyethylene (rotomolding grade) |
solid | 2.3027 |
| Silica (fused) | solid | 0.703 |
| Silver | solid | 0.233 |
| Sodium | solid | 1.23 |
| Steel | solid | 0.466 |
| Tin | solid | 0.227 |
| Titanium | solid | 0.523 |
| Tungsten | solid | 0.134 |
| Uranium | solid | 0.116 |
| Water at 100 °C (steam) | gas | 2.08 |
| Water at 25 °C | liquid | 4.1813 |
| Water at 100 °C | liquid | 4.1813 |
| Water at −10 °C (ice) | solid | 2.05 |
| Zinc | solid | 0.387 |
Table 1. List of various substances and their specific heat capacities.
Calorimetry
In calorimetry, a calorimeter is used to calculate how much heat is produced or absorbed during a chemical reaction.
>> calorimeter image
heat transfer :

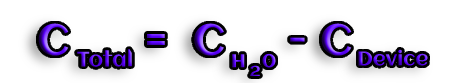
Example question :
In a calorimeter containing 1.20 kg of water, glucose is oxidized. What amount of heat will be emitted if the temperature rises from 19°C to 25.5°C degrees Celsius ?
Answer :
Cp(H2O) = 4.184 kJ . kg-1.K-1
Cdevice = 2.21 kJ.K-1
Cwater = 4.184 KJ .kg-1.K-1 * 1.20 kg = 5.02 kJ.K-1
Ctotal = Cdevice + Cwater = 7.23 kJ.K-1
Final Answer:
Q = Ctotal.(Tafter- Tbefore) = 7.23kJ.K-1 .(25.5°C - 19°C) = 47 kJ
Thermodynamics
The science of energy conversion during physical
and chemical processes is known as thermodynamics.
Using thermodynamics to foretell whether reactions would take place
spontaneously under specific circumstances is a crucial application in
chemistry.
Thermodynamic system
A system is viewed as a constrained, clearly defined
macroscopic spatial
region with specified parameters while
studying the principles of
thermodynamics.
Different interactions between a system and its environment are possible
depending on the nature of the system.
| Types of system | Mass | Work | Heat |
| Open | Yes | Yes | Yes |
| Closed | No | Yes | Yes |
| Adiabatic | No | Yes | No |
| Isochoric | No | No | Yes |
| Isolated | No | No | No |
Table 2. Different types of thermodynamic systems.
State functions
A thermodynamic systems macroscopic features are quantitatively described by state functions.
Examples:
-
Volume
-
Pressure
-
Temperature
As their name suggests, they are merely reliant on the system's current condition, regardless of the precise course that brought it about.
Equation of State
a formula for the relationship between state functions.
The ideal gas law is one such and is described
below :

p : pressure [Pa = N/m2]
V : volume [m3]
n : amount of substance [mol]
R : universal gas constant (8.314 J/mol.K)
T : absolute temperature [K]
Based on this formula we are able to measure the energy of a system : N/m => J => Energy
The 0th law of thermodynamics
They are in thermal equilibrium with one another
if two bodies A and B are in thermal equilibrium with body C.
This law can be used to build thermometers that measure a body's
temperature.
The 1st law of thermodynamics
For thermodynamic systems, an energy balance can be obtained using the energy conservation principle.
Consequently, an energetic state function is established, which represents the system's internal energy U.
As a state function, a system's core energy is only influenced by its current state and not by the steps that brought it about.
Consequently, there is a continual difference in the internal energy of two different states.
ΔU = U2 - U1
The first law of thermodynamics states that inner energy has a state function and that any change in inner energy equals the heat absorbed and the work performed by the system.
ΔU = Q - W
Sign conventions
Q>0 : system take up heat
Q<0 : system releases heat
W>0 : system does work
W<0 : work is done to the system
ΔU>0 : system takes up energy
ΔU<0 : system loses energy
Enthalpy is the sum of the system's internal energy and the product of its pressure and volume.
Typically, chemical reactions take place under a constant amount of pressure.
As a result, when a process involves mechanical work, the system expands in opposition to the applied pressure (e.g. if gases are produced)
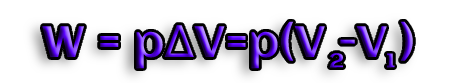
In this instance, we define the enthalpy change ΔH as:

substitution of ΔH for Q in ΔU = Q - W
ΔU = ΔH - W
ΔH=ΔU+W ; W=pΔV
ΔH=ΔU+pΔV
The enthalpy H is a state function, just like the inner energy U. It's outlined as:
H = U + pV
Once more, the difference in enthalpies between two states is constant and independent of path:
ΔH = H2 - H1
Solids and liquids have extremely minimal volume
changes, but if gases are involved in a chemical reaction, their volume
could change dramatically.
The change in material amount can be used to compute the difference in
volume if pressure and temperature are both constant:
p(V2-V1)=(n2-n1)RT <==> pΔV = ΔnRT
ΔH=ΔU+pΔV
ΔH=ΔU+ΔnRT
therefore we get the final formula :
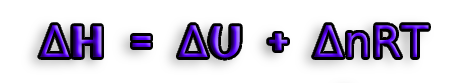
Example question :
The energy emitted by burning
CH4
was calculated in a calorimeter at 25°C under constant volume to be
-885.4 kJ/mol.
Calculate the reaction's ΔH.
Answer :
CH4 (g) + 202 (g) —> CO2 (g) + 2H2O (l) ΔU = -885.4 kJ/m0|
nR= 3 mol nP=1
ΔH = ΔU + ΔnRT
= -885.4 kJ/mol + (( 1-3 ).( 8.314-10-3 kJ/mol.K - 298.2 K ))
= -885.4 kJ/mol - 4.95 kJ/mol
= -890.4 kJ/mol
A reaction is called endothermic if ΔH > 0 and exothermic if ΔH < 0.
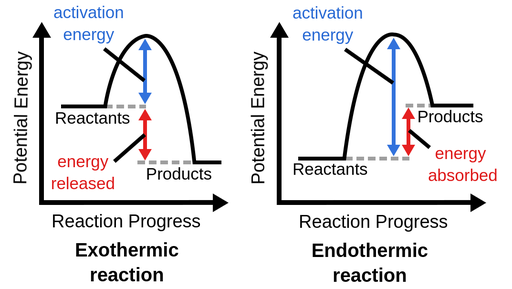
Figure 1. Heat is released during an exothermic reaction, raising the temperature of the immediate environment. An endothermic process removes heat from the environment and cools it.
Whether a change occurs in a single step or multiple steps, the enthalpy released or absorbed is the same in each case.
The law of energy conservation and Hess' law are compatible.
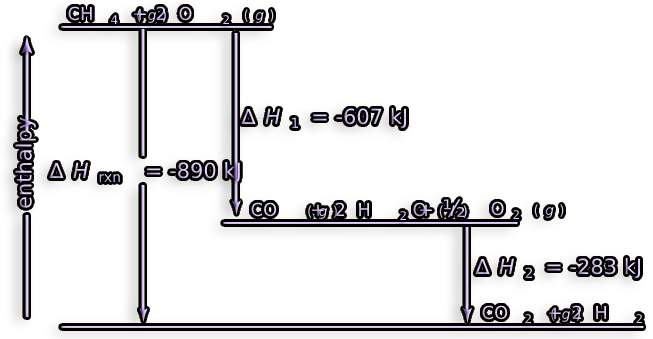
Energy creation would be conceivable if the net change in energy varied depending on the actions conducted, which is not possible.
According to Hess's law, as long as the beginning and final states of the reactants and products are the same, the change in enthalpy in a chemical reaction will be the same whether the reaction occurs in one step or multiple steps.
In other words, regardless of the method through which a chemical change happens, if a chemical change occurs by numerous different routes, the overall enthalpy change will be the same.
Even when it cannot be measured directly, Hess's law enables the calculation of the enthalpy change (H) for a reaction.

net change in enthalpy : ΔHnet
sum change in enthalpy reactions : ∑ΔHr
A net or overall equation is created by
combining chemical equations.
If the enthalpy changes for every equation in the series are known, the
enthalpy change for the net equation will be the total of those values.
The process is exothermic and is more likely to be spontaneous if the
net enthalpy change is negative, whereas positive H values are
associated with endothermic reactions.
Standard Enthalpy of Formation
The values of the standard enthalpy of formation
can be used to compute reaction enthalpies.
It is the change in enthalpy that occurs when an element is converted
into a 1 mol pure substance from its standard state*
Standard state* : i.e., the most stable form of the element at 1 bar [or, occasionally, 101.13 kPa] and a specific temperature, typically 25°C)
Symbol is ΔH0f
C(graphite) + O2(g) —> CO2(g) ΔH0f : 393.5kJ.mol-1
Just like this there are several standard enthalpies of formation unique to each substance.
Compute Enthalpies
From standard enthalpies of formation, the standard enthalpy of a reaction can be computed as follows:
ΔH° = ΔHf°(products) - ΔHf°(reactants)
Hydrogenation of ethene :
C2H4(g) + H2(g)
—> C2H6(g)
2C(graphite) + 2H2(g)
—>
C2H4(g)
ΔHf° =
52.30 kJ/mol
2C(graphite) + 3H2(g)
—>
C2H6(g)
ΔHf° =
-84.68 kJ/mol
C2H4(9) + H2(g)
—>
C2H6(g)
ΔH° =
-136.98 kJ/mol
Energy lattices and the Born-Haber Cycle
Keep in mind that ions create crystals. It is impossible to directly measure the energy released when creating a crystal from ions in the gas phase.
.png)
Figure 2. The lattice energy of NaCl is 787.3 kJ/mol, which is just somewhat less than the energy released during the combustion of natural gas. When the ions are tiny, the binding between ions with opposite charges is strongest.
It can be computed from other empirically determined values using Hess' law.
The Born-Haber cycle is the name of this approach.
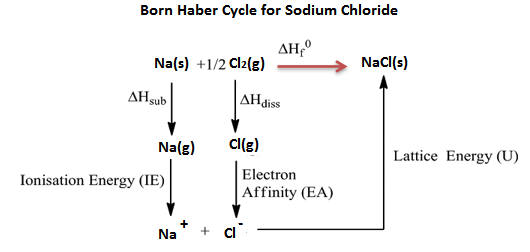
Figure 2. The born haber cycle for NaCl.
A Born Haber cycle is a series of enthalpy changes that results in the production of a solid, crystalline ionic compound from elemental atoms in their standard state, with the net enthalpy of the solid compound decreasing to zero.
Born Haber cycle for NaCl:
Step 1: Sublimation of metallic sodium of gaseous sodium atom with the enthalpy of sublimation = △Hs
Na(s)→Na(g);△H=△Hs
Step 2 : Dissociation of molecule of chlorine to chlorine atoms with enthalpy of dissociation = △Hd
1/2Cl2(g)→Cl(g);△H=△H
Step 3: Ionization of gaseous sodium with the enthalpy of ionization = △Hi
Na(g)→Na+ (g)
Step 4: Addition of electron to gaseous chlorine atom with the enthalpy of electron affinity = △HEa
Cl (g) +e−→Cl−(g),△H=△HEa
Step 5: Close packing of gaseous sodium ion and chloride ion to form the lattice structure of NaCl, with lattice chloride ion to form a lattice structure of NaCl with Lattice energy = U.
NaCl+(g)+Cl−(g)→NaCl△;△H=U
Step 6: But the sum of all the energies will be equal to the heat of formation of one mole of sodium chloride from its reluctant i.e, △Hf
Na(s)+1/2Cl2 (g)→NaCl(s);△H=△Hf
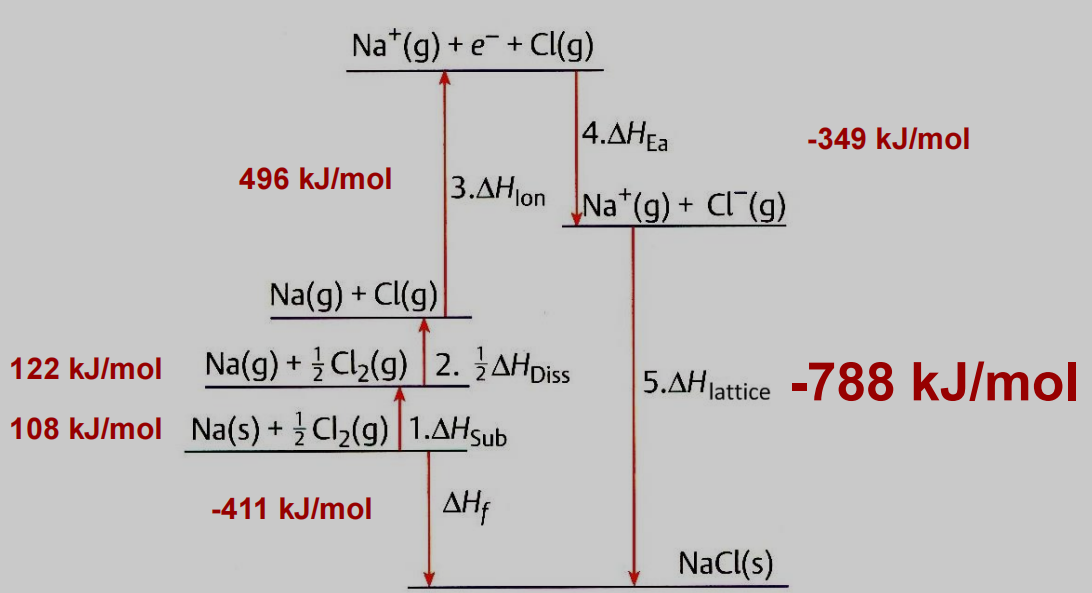
Figure 3. The overall born haber cycle for NaCl.
2nd Law of Thermodynamics
Energy must be conserved during chemical and physical processes, according to the first law.
The second law of
thermodynamics asserts that heat cannot move from a reservoir of lower
temperature to a reservoir of higher temperature in a cyclic process.
Even yet, we are unsure of which processes will be triggered
spontaneously and which won't.
The entropy S, a new state function introduced by the second law of
thermodynamics, allows us to predict whether a reaction will be
spontaneous or not.
Entropy
One way to think of a system's entropy is as a measure of its disorder; the higher the entropy, the lesser the system's order.
.png)
Figure 4. The overall entropy os a sysytem is linked to the order and disorder of the system.
Entropy will rise in a spontaneous (naturally occurring) process.
The highest order and least entropy are found in the solid, crystalline state of a given substance. The liquid state has an intermediate entropy, and the gas state has the maximum entropy.
Melting and vaporization: entropy increases
Condensation and freezing: entropy reduction
Things shouldn't spontaneously condense or freeze if we say the second law would only apply to a specific system.
As a result, we also need to take the environment's and the system's entropy changes into account:
ΔStot = ΔSsys + ΔSenv
Energy is released (negative enthalpy) when a liquid freezes and is absorbed by the surroundings. This makes the environment more entropic.
Always take into account the overall change in entropy.
Heat emitted by the system is what causes the environment's entropy to alter.
The environment's change in entropy for a process occurring at constant pressure and temperature can be computed as follows:

entropy units are : J/K
Gibbs Free energy
ΔSenv = ΔH/T
ΔSenv = ΔStot +ΔSsys
ΔStot +ΔSsys = ΔH/T
TΔStot + TΔSsys =ΔH
Substitute ΔG for -TΔStot :
ΔG + TΔSsys= ΔH
ΔG = ΔH-TΔSsys
ΔG = ΔH-TΔS changes in gibbs free energy
G = H-TS gibbs free energy
Meaning of ΔG :
G is like H and S- a state function ΔG = G2-G1
Finally, the Gibbs free energy function allows us to determine whether a process will occur spontaneously under specific conditions (temperature and pressure constant)
ΔG > 0 reaction will not happen spontaneously.
ΔG = 0 System is in thermodynamic equilibrium.
ΔG < 0 reaction will happen spontaneously.
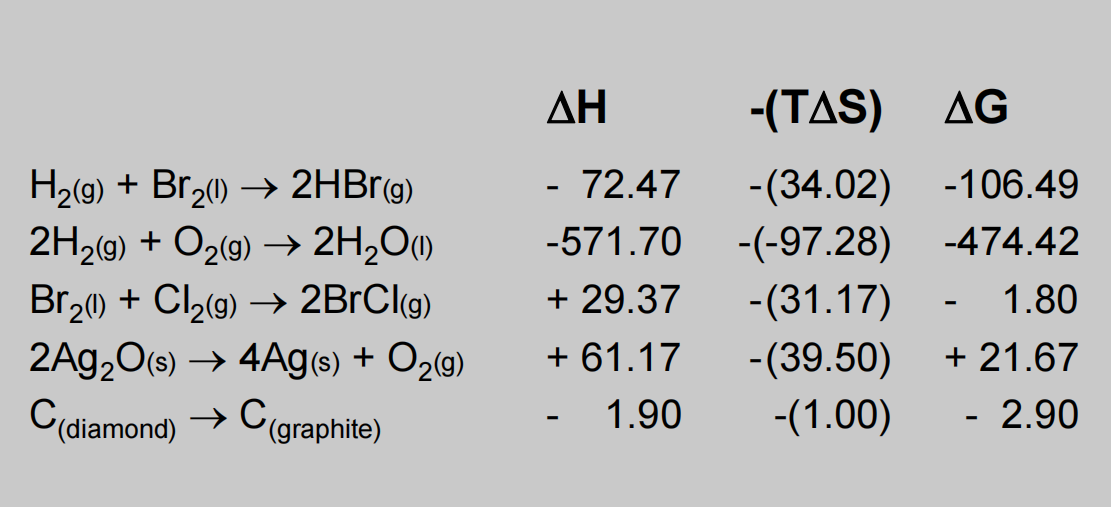
Figure 5. Some reactions with respect to gibbs free energy.
Standard Gibbs Energy of Formation
Gibbs free energy change that occurs when 1 mole of a substance is formed from its constituent elements in their standard states (often at 1 bar and 25°C).
3rd Law of Thermodynamics
As the absolute temperature gets closer to zero, the entropy of a perfect crystal decreases.
The third law of thermodynamics states that the entropy of a system
approaches a constant value as the temperature approaches absolute zero.
The entropy of one mole of substance at standard conditions (often 1
bar/25°C) is known as the standard molar entropy S0.
For a reaction, the modification of the standard molar entropy is
referred to as ΔS0.
Example question :
Vaporization of methanol — calculate the normal boiling point of methanol.
H3COH(I) <==> H3COH(g)
ΔH0 = 37.4 kJ/mol
ΔS0 = 111 J/(mol.K)
The related temperature for "ΔG = 0" is the same as methanol's typical boiling point. We consider "ΔH" and "ΔS" to be temperature-independent.
ΔG0 = ΔH0-TΔS0 = 0
37.4 kJ/mol - T . 0.111 kJ/(mol-K) = 0
T = (37.4 kJ/mol) / (0.111 kJ/(mol.K) ) = 337 K (64°c)
Equilibrium and Free Energy
Fundamentals: Equilibrium must be reached for
any thermodynamic system.
If they reduce the Gibbs free energy, the system changes.
The Gibbs free energy is lowered by an increase in entropy and a
decrease in enthalpy.
---- Summary ----
As of now you know all basics of all the laws of thermodynamics.
-
1st law of thermodynamics
-
2nd law of thermodynamics
-
3rd law of thermodynamics
-
etc..
________________________________________________________________________________________________________________________________

________________________________________________________________________________________________________________________________
